Organoids
Organoids
The successful cultivation of the first small intestinal organoid from Lgr5+ intestinal stem cells by Hans Clevers in 2009 ushered in the era of organoid research. After that, organoids have become popular in the scientific research circle and become a hotspot in vitro model system in just over a decade. Herein, we will describe the development history, construction & preparation, categories, and applications of organoids.
1. What Are Organoids?
As the name implies, an organoid resembles a real represent organ. Scientifically speaking, organoids are in vitro generated three-dimensional (3D), mini-cluster of cells that highly simulate the structure and function of the corresponding organ in vivo [1]. More colloquially, an organoid is a mini-organ that is generated in vitro with the ability to self-renew and self-organize and performs organ functions similar to those of the tissue of origin.
Three Characteristics of Organoids:
- Cells are capable to self-organize via cell sorting and spatially restricted lineage commitment in a manner similar to the organ of origin;
- Contain more than one cell type identical to the organ of origin [2];
- Can recapitulate some specific functions of the organ of origin, such as filtration, excretion, neural activity, and contraction [2].
2. The Developmental History of Organoids
Back in 1907, Henry Van Peters Wilson found that mechanically dissociated sponge cells could reaggregate and self-organize into new normal functional sponge organisms [3].
In the following decades, several similar dissociation-reaggregation patterns were observed in vertebrate models, such as Holtfreter's experiment in amphibian pronephros in 1944 [4] and Weiss's experiment in chick embryos in 1960 [5].
Differentiation of embryoid bodies in vitro was observed in 1961 by Pierce and Verney [6].
Steinberg formulated the Differential Adhesion Hypothesis (DAH) of cell sorting out in 1964.
Pluripotent stem cells (PSCs) were first isolated and established from mouse embryos in 1981 [7] [8].
Stem cell research began to flourish. The improvement of cell culture conditions through mimicking the in vivo microenvironment was made in 1987. Li ML et al. demonstrated that breast epithelia can organize into 3D ducts and ductules in EHS (Engelbreth-Holm-Swarm) ECM (extracellular matrix) extract [9]. Shannon JM et al. discovered the differentiation of alveolar type II epithelial cells in ECM extract [10].
It was not until 1998 that American biologist James Thomson isolated and cultured human embryonic stem cells from human blastocysts for the first time [11].
Human-induced pluripotent stem cells (iPSCs) were successfully produced by reprogramming mouse and human fibroblasts in 2006, which has had a major impact on stem cell and organoid research [12].
In 2008, Sasai et al. laid the foundations of brain organoids by demonstrating the self-organization of human brain iPSCs into neural cells that formed polarized cortical tissues [13].
In 2009, Hans Clevers and colleagues first demonstrated that single Lgr5+-expressing intestinal adult stem cells (ASCs) could self-organize and differentiate to form intestinal crypt-villus structures that contained all intestinal cell types [14]. This ushered in a new era in the development of organoid technology. Thereafter, organoids were constructed and employed as a novel research model that could be used instead of traditional cell lines and heterogeneous animal models.
In 2010, renal organoids were produced from murine fetus-derived kidney stem cells [15]. Gut organoids were generated from human PSCs in vitro.
Nakano et al. demonstrated that a self-organized optic cup structure was formed from human PSCs in 3D culture in 2012.
Human brain organoids are generated from iPSCs derived from cells from patients with microcephaly in 2013 [16]. Lee JH et al. revealed that lung organoids can be produced from the 3D coculturing of endothelial cells and adult bronchioalveolar stem cells [17].
The mammary gland, fallopian tube, and hippocampal organoids were created in 2015.
Snake venom gland organoids were generated in 2020 [18].
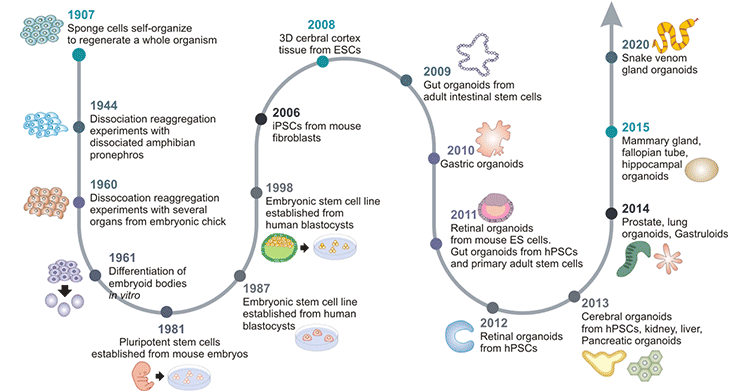
Figure 1. Timeline for the development of organoid cultures
This picture is cited from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7468890/
3. How Are Organoids Made?
Organoids can be generated from two types of stem cells: pluripotent stem cells (PSCs), such as embryonic stem cells (ESCs) and induced pluripotent stem cells (iPSCs), and organ-restricted adult stem cells (AdSCs). These cells are cultured in a specific environment that allows them to follow the ingrained genetic instructions to self-organize into functional 3D structures.
The methods for culturing organoids from various tissues are similar. Stem cells are most frequently cultured in Matrigel and in the presence of suitable exogenous factors including chemical small molecule inhibitors/activators, cytokines, and medium additives, and can be coaxed into forming organoids of corresponding organs. The preparation of different organoids requires different combinations of additives. Even for tissues with very similar structures such as the small intestine and colon, the combinations of additives required for the preparation of organoids are different. In addition, generating organoids directly derived from patients' tumors is also a practical approach.
Three Elements of Organoid Construction
- physical characteristics of the culture environment
- activation or inhibition of key signaling pathways
- the initial cell type and condition
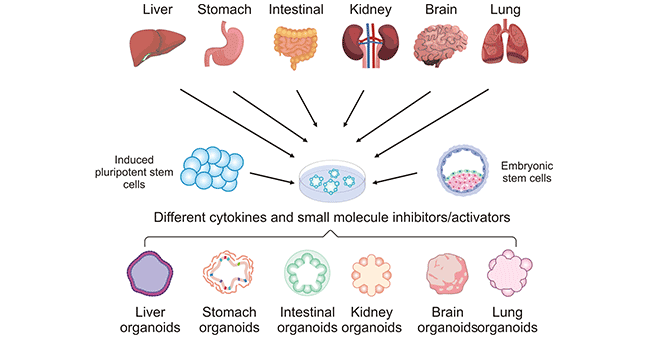
Figure 2. Establishment process of different human organoids
The picture is cited from: https://journals.plos.org/plospathogens/article?id=10.1371/journal.ppat.1010080
4. Classification of Organoids
Since the successful establishment of epithelial organoids in 2009, organoid cultures have been used in various organs, including the brain, inner ear, lung, liver, colon, liver, pancreas, small intestine, prostate, stomach, and breast.
5. Applications
Although the wide application of organoid technology in the research community is still in its infancy, organoid technology has great potential as a tool for studying a wide range of subjects, including developmental biology, disease pathology, cell biology, regeneration mechanisms, precision medicine, and drug toxicity and efficacy tests.
6. CUSABIO Featured Products Related to Organoids
References:
[1] Clevers, H. Modeling Development and Disease with Organoids [J]. Cell 2016, 165, 1586–1597.
[2] Stevens KR, Kreutziger KL, Dupras SK et al. (2009) Physiological function and transplantation of scaffold-free and vascularized human cardiac muscle tissue [J]. Proc Natl Acad Sci 106:16568–16573.
[3] Wilson HV. A new method by which sponges may be artificially reared [J]. Science 25: 912–915, 1907.
[4] Holtfreter, Johannes. Experimental studies on the development of the pronephros [J]. Rev. can. biol. 3 (1943): 220-250.
[5] Weiss, Paul, and A. C. Taylor. Reconstitution of complete organs from single-cell suspensions of chick embryos in advanced stages of differentiation [J]. Proceedings of the National Academy of Sciences of the United States of America 46.9 (1960): 1177.
[6] Pierce, G. B., Jr. & Verney, E. L. (1961). An in vitro and in vivo study of differentiation in teratocarcinomas [J]. Cancer 14, 1017-1029.
[7] Martin GR. Isolation of a pluripotent cell line from early mouse embryos cultured in medium conditioned by teratocarcinoma stem cells [J]. Proc Natl Acad Sci USA 78: 7634–7638, 1981.
[8] Evans M. J., Kaufman M. H. 1981. Establishment in culture of pluripotential cells from mouse embryos [J]. Nature 292, 154–156.
[9] Li ML, Aggeler J, Farson DA, et al. Influence of a reconstituted basement membrane and its components on casein gene expression and secretion in mouse mammary epithelial cells [J]. Proc Natl Acad Sci USA 84: 136–140, 1987.
[10] Shannon JM, Mason RJ, Jennings SD. Functional differentiation of alveolar type II epithelial cells in vitro: effects of cell shape, cell-matrix interactions and cell-cell interactions [J]. Biochim Biophys Acta 931: 143–156, 1987.
[11] Thomson JA, Itskovitz-Eldor J, et al. Embryonic stem cell lines derived from human blastocysts [J]. Science 282: 1145–1147, 1998.
[12] Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors [J]. Cell 126: 663–676, 2006.
[13] Eiraku, Mototsugu, et al. Self-organized formation of polarized cortical tissues from ESCs and its active manipulation by extrinsic signals [J]. Cell stem cell 3.5 (2008): 519-532.
[14] Hans Clevers, Sato T, Vries RG, et al. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche [J]. Nature 459: 262–265, 2009.
[15] Unbekandt, Mathieu, and Jamie A. Davies. Dissociation of embryonic kidneys followed by reaggregation allows the formation of renal tissues [J]. Kidney international 77.5 (2010): 407-416.
[16] Lancaster, Madeline A., et al. Cerebral organoids model human brain development and microcephaly [J]. Nature 501.7467 (2013): 373-379.
[17] Lee, Joo-Hyeon, et al. Lung stem cell differentiation in mice directed by endothelial cells via a BMP4-NFATc1-thrombospondin-1 axis [J]. Cell 156.3 (2014): 440-455.
[18] Clevers, H. Modeling Development and Disease with Organoids [J]. Cell 2016, 165, 1586–1597.
